Abstract
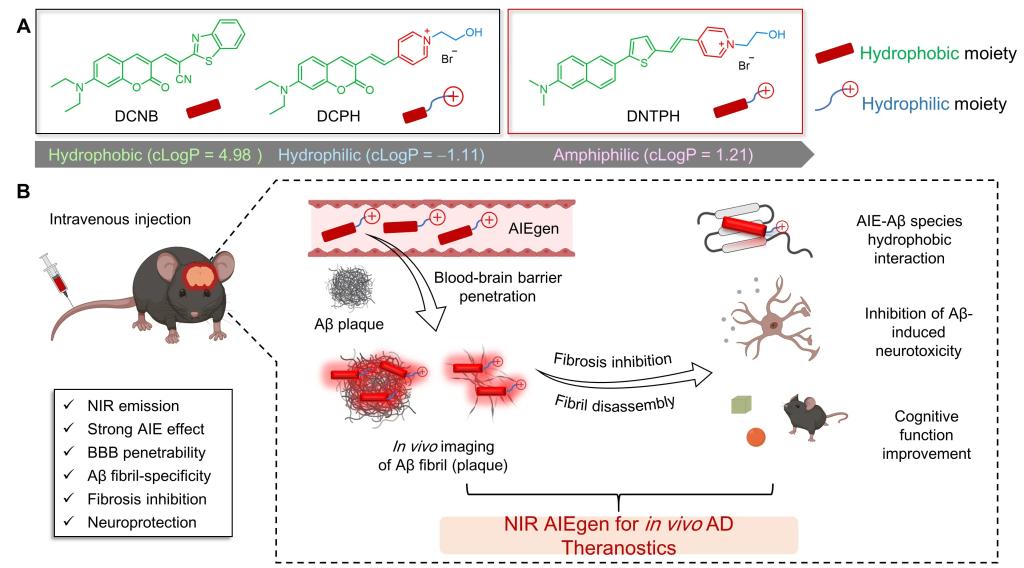
Optimized theranostic strategy for Alzheimer’s disease (AD) remains almost vacant from bench to clinic. Current probes and drugs attempting to prevent β-amyloid (Aβ) fibrosis encountered failures due to the blood-brain barrier (BBB) penetration challenge and blind intervention time window. Herein, we designed a near-infrared (NIR) aggregation-induced emission (AIE) probe, DNTPH, via balanced hydrophobicity-hydrophilicity. DNTPH binds selectively to Aβ fibrils with a high signal-to-noise ratio. In vivo imaging revealed its excellent BBB permeability and long-term tracking ability with high-performance AD diagnosis. Remarkably, DNTPH exhibited a strong inhibitory effect on Aβ fibrosis and fibril disassembly thereby attenuating Aβ-induced neurotoxicity. DNTPH treatment significantly reduced Aβ plaques and rescued learning deficits in AD mice. Thus, DNTPH serves as the first AIE in vivo theranostic agent for real-time NIR imaging of Aβ plaques and AD therapy simultaneously.