Abstract
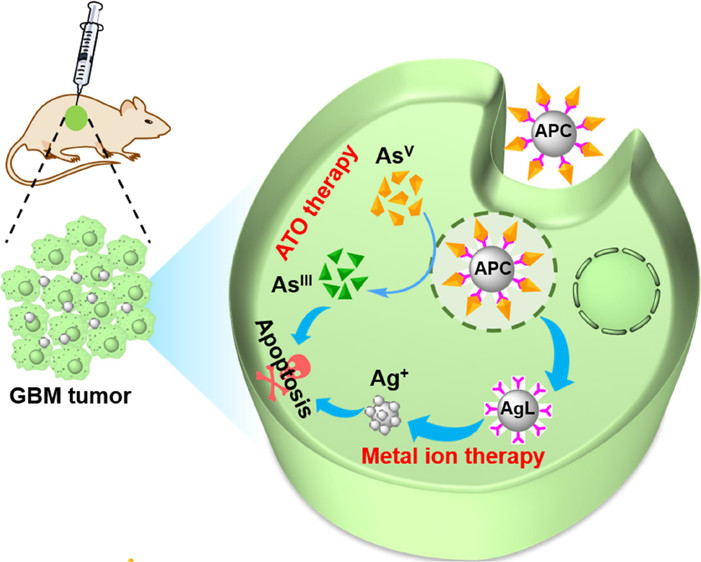
Glioblastoma (GBM) has a distinct internal environment characterized by high levels of glutathione (GSH) and low oxygen partial pressure, which significantly restrict most drugs’ effectiveness. Arsenic-based drugs are emerging candidates for treating solid tumors; however, relatively high doses in solo systems and inconsistent complementary systems severely damage the normal tissues. We proposed a novel covalently conjugated strategy for arsenic-based therapy via arsenic-boronic acid complex formation. The boronic acid was modified on silver (AgL) to capture AsV under an alkaline condition named arsenate plasmonic complex (APC) with a distinct Raman response. The APC can precisely release the captured AsV in lysosomal acidic pH that specifically targets TME to initiate a multimodal therapeutic effect such as GSH depletion and reactive oxygen species generation. In addition, GSH activation leads to subconverted AsV into AsIII, which further facilitated glutathione peroxidase (GPx) and superoxide dismutase inhibition, whereas the tumor selective etching of the silver core triggered by endogenous H2O2 that can oxidize to generate highly toxic Ag ions produces and supplies O2 to help the alleviated hypoxia. Both in vitro and in vivo data verify the APC-based chemotherapy paving the way for efficient nanomedicine-enabled boronate affinity-based arsenic chemotherapeutics for on demand site-specific cancer combination treatment of GBM tumors.