Abstract
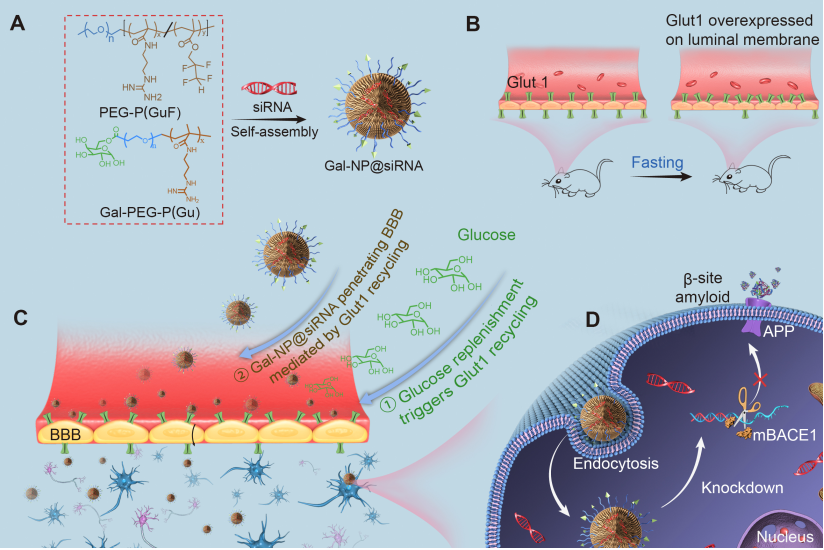
Toxic aggregated amyloid-β accumulation is a key pathogenic event in Alzheimer’s disease (AD), which derives from amyloid precursor protein (APP) through sequential cleavage by BACE1 (β-site APP cleavage enzyme 1) and γ-secretase. Small interfering RNAs (siRNAs) show great promise for AD therapy by specific silencing of BACE1. However, lack of effective siRNA brain delivery approaches limits this strategy. Here, we developed a glycosylated “triple-interaction” stabilized polymeric siRNA nanomedicine (Gal-NP@siRNA) to target BACE1 in APP/PS1 transgenic AD mouse model. Gal-NP@siRNA exhibits superior blood stability and can efficiently penetrate the blood-brain barrier (BBB) via glycemia-controlled glucose transporter-1 (Glut1)–mediated transport, thereby ensuring that siRNAs decrease BACE1 expression and amyloid plaques, suppress phosphorylated tau protein production, and promote myelin regeneration. Noticeably, Gal-NP@siBACE1 administration restored the deterioration of cognitive capacity in AD mice without significant side effects. This novel “Trojan horse” strategy of delivering siRNA through BBB supports the utility of RNA interference therapy in neurodegenerative diseases.