Abstract
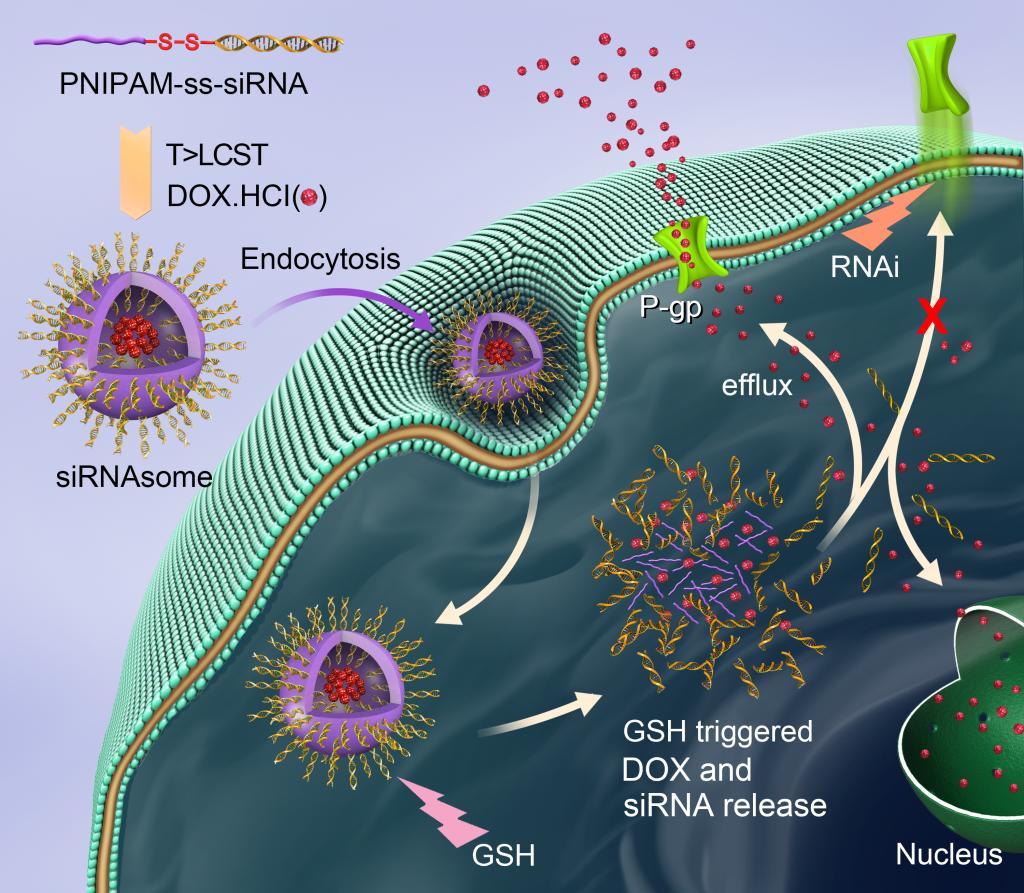
Nanoparticles show great potential for drug delivery. However, suitable nanostructures capable of loading a range of drugs (including hydrophobic/hydrophilic small molecules or biomolecular agents) together with the codelivery of siRNAs that avoid the problem of cation-associated cytotoxicity, are lacking. Herein, we report a novel, siRNA-based vesicle (siRNAsome) nanostructure which consists of a hydrophilic siRNA shell, a thermal and intracellular reduction sensitive hydrophobic median layer, and an empty aqueous interior that meets this need. The siRNAsome can serve as a versatile nanostructure to load drug agents with divergent chemical properties, therapeutic proteins as well as codelivering immobilized siRNAs without transfection agents. Importantly, a particular advantage of our siRNAsome is that inherent thermal/reduction responsiveness enables it to control drug loading and release. We show that when siRNAsomes are loaded with the hydrophilic drug doxorubicin hydrochloride (Dox·HCl) and anti-P-glycoprotein (Pgp) siRNA (to target the Pgp drug exporter), synergistic therapeutic activity is achieved in multidrug resistant (MDR) cancer cells and tumor model.
http://dx.doi.org/10.1002/ange.201814289
