Abstract
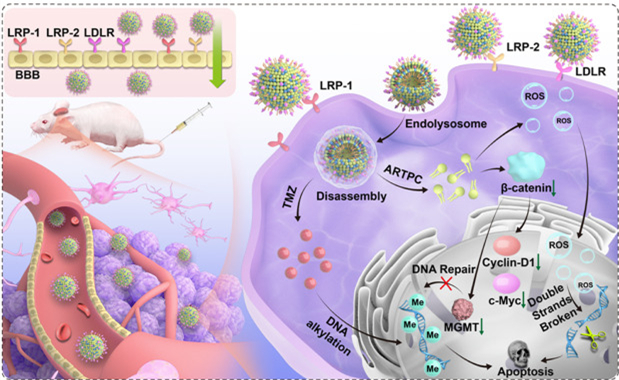
The effective treatment of glioblastoma (GBM) is a great challenge because of the blood-brain barrier (BBB) and the growing resistance to single-agent therapeutics. Targeted combined co-delivery of drugs could circumvent these challenges; however, the absence of more effective combination drug delivery strategies presents a potent barrier. Here, a unique combination ApoE-functionalized liposomal nanoplatform based on artesunate-phosphatidylcholine (ARTPC) encapsulated with temozolomide (ApoE-ARTPC@TMZ) was presented that can successfully co-deliver dual therapeutic agents to TMZ-resistant U251-TR GBM in vivo. Examination in vitro showed ART-mediated inhibition of DNA repair through the Wnt/β-catenin signaling cascade, which also improved GBM sensitivity to TMZ, resulting in enhanced synergistic DNA damage and induction of apoptosis. In assessing BBB permeation, the targeted liposomes were able to effectively traverse the BBB through low-density lipoprotein family receptors (LDLRs)-mediated transcytosis and achieved deep intracranial tumor penetration. More importantly, the targeted combination liposomes resulted in a significant decrease of U251-TR glioma burden in vivo that, in concert, substantially improved the survival of mice. Additionally, by lowering the effective dosage of TMZ, the combination liposomes reduced systemic TMZ-induced toxicity, highlighting the preclinical potential of this novel integrative strategy to deliver combination therapies to brain tumors.